The Posterior Cingulate Cortex Again Forgotten
Koch et al., (2022) 'Repetitive TMS applied to the precuneus stabilizes cognitive status in Alzheimer's disease,' we think that some findings are misunderstood and there are methodological problems. Neuromodulation approaches have been investigated for a long time in the treatment of Alzheimer's dementia. On the other hand, the precuneus region, which is the subject of the research, cannot be considered as an isolated region due to its close neighborhood. Precuneus, it is considered the main center of the default mode network (DMN) like the dorso-lateral prefrontal cortex (DLPFC). To date, many DLPFC stimulation studies have been performed with repetitive transcranial magnetic stimulation (rTMS) has been published. It is difficult to evaluate the effect claimed as a result of the research specific to the precuneus region. Theoretically, the effect can be expected with the excitation of any part of the default network. However, unlike previous rTMS studies that stimulated the DLPFC, the authors chose to stimulate the precuneus, the main center of DMN.
Precuneus, anatomically medial part of the parietal lobes. Immediately in the lower neighborhood posterior cingulate cortex (PCC). PCC is a large node within the DMN. It has intense structural connectivity to other brain regions. The main problem in this study is how they protect the PCC from stimulation or isolate the precuneus that sits on the PCC alone. Anatomically speaking, it is impossible to stimulate the precuneus by targeting with rTMS alone. PCC has one of the highest levels of metabolism in the brain (Gosseries et al., 2014). PCC, on the other hand, differs from the precuneus, which is not part of the cingulate cortex but is functionally tightly related (Leech et al., 2012; Vogt et al., 2006). In addition, in relation to the underlying Alzheimer's pathology, PCC has higher acetylcholine receptor binding densities than the anterior cingulate cortex (ACC). The ventral PCC and the dorsal PCC mutually connect with the precuneus. There are connections between various sensory areas, such as the temporal area, V3 and the auditory association cortex (Palomero et al., 2008; Vogt and Laureys, 2009). The PCC has been repeatedly observed to be one of the few brain regions that show consistent reductions or 'deactivations' in hemodynamic activity in a wide variety of attention-demanding tasks.
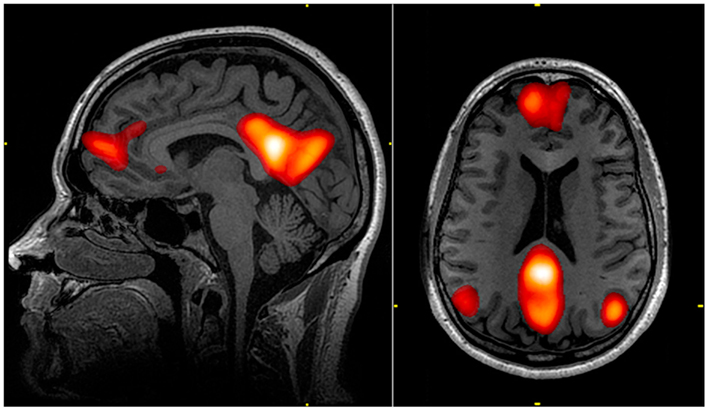
PCC hemodynamic activation occurs during tasks that rely on episodic memory, navigation, self-referential cognition, or abstract thought. PCC activity is typically suppressed during episodic memory encoding and increases during episodic memory retrieval. The PCC functions as a hub connecting reward possibilities with broader contextual schemas that rely on or operate on recall processes. Again, meta-analyses have shown striking consistency of dorsal PCC involvement during decision making (Foster et al., 2022). In particular, the dorsal PCC is involved in tasks that involve balancing attention between internal/mnemonic and external/perceptual sources and responding to the familiarity of a stimulus even outside of explicit memory contexts. Ventral PCC is a semantic-memory processing network (medial temporal lobe and inferior parietal lobe) as well as the representation of details about himself and others. Interestingly, the putative location and face 'domains' of the PCC show particularly strong responses when a memory aspect is included in the responses (compared to unfamiliar stimuli) during perception of personally familiar stimuli. Ventral PCC includes identification of positive and negative objects and events, scaling of common values, decision-mediated feedback on predicted outcomes, and identification of personal relevance of objects and contexts (Vogt and Laureys, 2009).
Intracingulate connection (cingulum bundle) between the ACC and the ventral PCC may involve joint activation of both. The connection between the ACC and the ventral PCC is even tighter when considering other cortical connections. It probably reflects the PCC's role in maintaining a broad focus of attention. Subregions within the PCC interact with the attention, language, and DMN that are very close within the PCC.

Human PCC showed suppression or deactivation of high-frequency activity during externally directed tasks such as target detection, visual search, mental calculation, and sustained attention. In intracranial recordings, the intrinsic oscillatory activity of PCC is in the theta band range (4-7 Hz), unlike the activity of neighboring brain regions in the occipital alpha rhythm (8-10 Hz) at rest. In addition, PCC theta oscillations exhibit cross-frequency coupling with high frequencies (70-150 Hz). This activity is similar to that of the hippocampus and potentially the DMN. Theta synchronization between the temporal lobe cortex occurs during autobiographical recall, prior to high-frequency activation within the ventral PCC (Leech et al., 2012). Such findings suggest that ventral PCC medial provides support for the hypothesis that the temporal lobe is closely related to recall functions. From this perspective, the high-frequency matching source that the researchers detected may not be the precuneus alone.
Alzheimer's disease severely affects the cingulate cortex. It has a profound and early impact, especially in PCC. The initial sites of action of mild cognitive impairment are in the dorsal PCC domain. These areas are those involved in self-related visuospatial orientation and sensorimotor orientation, respectively. Considering these functions, it is not possible to evaluate PCC functions anatomically and functionally separately from the precuneus. On the other hand, if researchers reanalyze their results according to the visual-spatial perception subscores of the scales, not according to the global scale scores at 24 weeks, different results may be revealed.
References
Koch G, Paolo CE, Bonnì S, Borghi I, Assogna M, Minei M, Pellicciari MC, Motta C, D’Acunto A, Porrazzini F. Precuneus magnetic stimulation for Alzheimer’s disease: a randomized, sham-controlled trial. Brain 2022;145(11):3776–3786
Gosseries O, Di H, Laureys S and Boly M. Measuring Consciousness in Severely Damaged Brains. Annual Review of Neuroscience 2014;37(1):457-478.
Leech R, Braga R and Sharp DJ. Echoes of the Brain within the Posterior Cingulate Cortex. Journal of Neuroscience 2012;32(1): 215-222.
Vogt BA, Vogt LJ, Laureys S. Cytology and functionally correlated circuits of posterior cingulate areas. NeuroImage 2006; 26:452-466.
Palomero-Gallagher N, Mohlberg H, Zilles K & Vogt B. Cytology and receptor architecture of human anterior cingulate cortex. J Comp Neurol 2008; 508:906-926.
Vogt BA, Laureys S. The primate posterior cingulate gyrus: connections, sensorimotor orientation, gateway to limbic processing. B.A. Vogt (Ed.), Cingulate neurobiology and disease, Oxford University Press, Oxford (2009), pp. 275-308
Foster BL, Koslov SR, Aponik-Gremillion L et al. A tripartite view of the posterior cingulate cortex. Nat Rev Neurosci 2022. https://doi.org/10.1038/s41583-022-00661-x
Sultan Tarlacı, Editor
DOI: 10.5281/zenodo.7808750
















